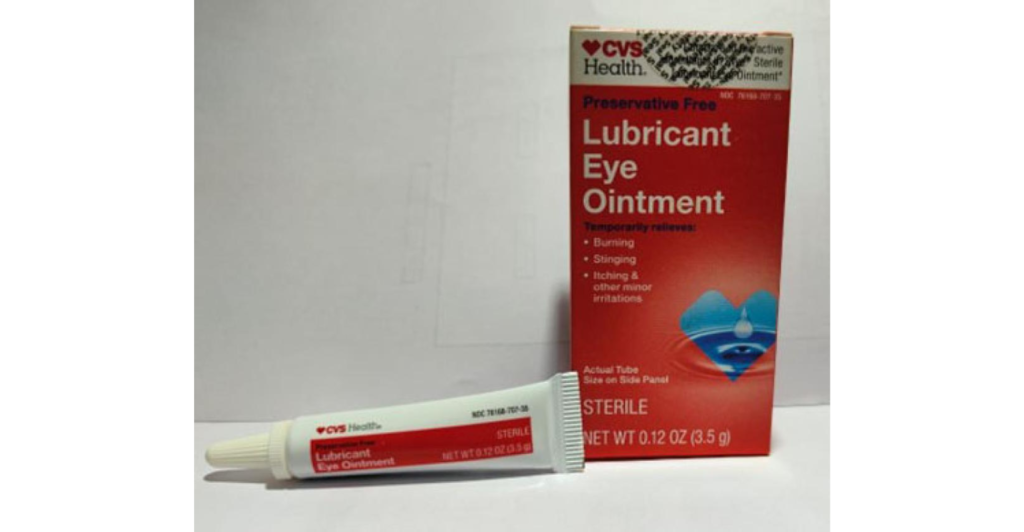
The Food and Drug Administration announced that Brassica Pharma Pvt. Ltd. has voluntarily recalled four eye ointment products sold at Walmart, CVS Pharmacy, and elsewhere, due to the potential risk for eye infections and other harm.
The FDA released a statement on Monday noting that on Feb. 12, the company announced it was recalling Equate Lubricant Eye Ointment, Equate Style Lubricant Eye Ointment, CVS Health Lubricant Eye Ointment, and Lubricant PM Ointment.
The Thane, Maharashtra, India-based company said in the press release that the products, which have expiration dates ranging from February 2024 to September 2025, were recalled “due to a lack of sterility assurance at the facility” during an FDA inspection.
“For those patients who use these products, there is a potential risk of eye infections or related harm,” the company said. “These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.”
As of Feb. 16, Brassica had not received any reports of adverse events related to the recall, the company said. The products are distributed across the U.S. to Walmart, CVS, AACE Pharmaceuticals Inc., other retailers, and wholesalers.
“These distributors shall be further notifying the wholesalers and retailers via mail of this voluntary recall and arranging for return of all impacted products…,” the Brassica press release noted. “Consumers, distributors, and retailers that have any product which is being recalled should cease distribution of the product. Consumers should stop using the recalled eye ointment and may return any of the above listed products to the place of purchase.”
Questions concerning the recall can be directed to Brassica Pharma Pvt. Ltd. at +1 833-225-9564 or [email protected].
The Food and Drug Administration announced that Brassica Pharma Pvt. Ltd. has voluntarily recalled four eye ointment products sold at Walmart, CVS Pharmacy, and elsewhere, due to the potential risk for eye infections and other harm.
The FDA released a statement on Monday noting that on Feb. 12, the company announced it was recalling Equate Lubricant Eye Ointment, Equate Style Lubricant Eye Ointment, CVS Health Lubricant Eye Ointment, and Lubricant PM Ointment.
The Thane, Maharashtra, India-based company said in the press release that the products, which have expiration dates ranging from February 2024 to September 2025, were recalled “due to a lack of sterility assurance at the facility” during an FDA inspection.
“For those patients who use these products, there is a potential risk of eye infections or related harm,” the company said. “These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.”
As of Feb. 16, Brassica had not received any reports of adverse events related to the recall, the company said. The products are distributed across the U.S. to Walmart, CVS, AACE Pharmaceuticals Inc., other retailers, and wholesalers.
“These distributors shall be further notifying the wholesalers and retailers via mail of this voluntary recall and arranging for return of all impacted products…,” the Brassica press release noted. “Consumers, distributors, and retailers that have any product which is being recalled should cease distribution of the product. Consumers should stop using the recalled eye ointment and may return any of the above listed products to the place of purchase.”
Questions concerning the recall can be directed to Brassica Pharma Pvt. Ltd. at +1 833-225-9564 or [email protected].