Cold-cloud precipitation and super-cooled water

Glacier FarmMedia – Preliminary numbers for October’s global temperatures are coming out and they don’t look good.
Read Also
Future of high horsepower could be diesel, with a boost
Practicality now drives engine and power innovation in agriculture equipment, with companies finding solutions that make more sense on the…
According to the Copernicus Climate Change Service, operated by the European Centre for Medium-Range Weather Forecasts, October 2023 was the warmest October on record globally, with an average surface air temperature of 15.3 C, which was 0.85 C above the 1991-2020 average for October and 0.4 C above the previous warmest October in 2019.
This was the second greatest monthly anomaly behind September 2023, and was 1.7 C warmer than an estimate of the October average for 1850-1900, the designated pre-industrial reference period.
For 2023 to date, the global mean temperature is the highest on record at 1.43 C above the 1850-1900 pre-industrial average, and 0.1 C higher than the 10-month average for 2016, which is currently the warmest calendar year on record.
This year now has more than a 99 per cent chance of being the warmest on record. Oceans continue at record-warm levels so we may see even warmer global temperatures in 2024.
Despite the warm late summer and early fall we’re heading into winter, when I get lots of emails asking about the ‘mixed bag’ of precipitation we experience in many parts of Canada — snow, freezing rain, rain, then back to snow.
There are two types of clouds: cold and warm. A warm cloud is any cloud that forms and exists in temperatures above freezing. A cold cloud is any cloud that has at least some part below the freezing point.
Before we look at the precipitation process in cold clouds, we need to explore the idea of super-cooled water. In most occurrences of freezing rain, we find temperatures just slightly below 0 C. This means the surfaces to which the raindrops freeze are just a little below 0 C.
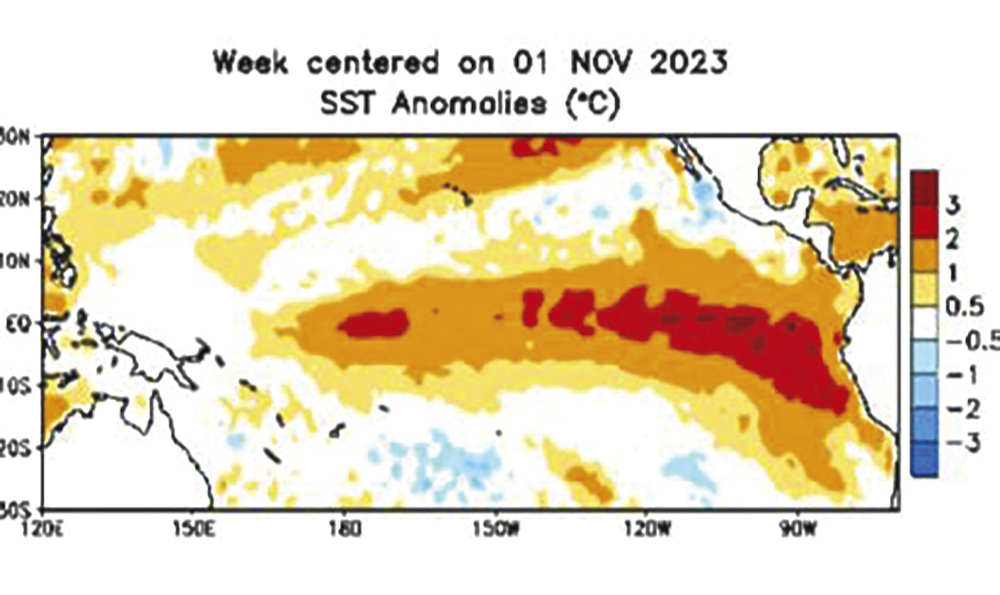
photo:
Copernicus Climate Change Service
If you’ve dropped cold water on a freezing surface, you know the water does not freeze instantly. So, why does a raindrop freeze as soon as it hits a solid surface? That falling raindrop was super-cooled: the water in the raindrop is below the freezing point, yet still liquid.
How is this possible? We all learned in school that water behaves differently than most other substances on Earth. While most substances are most dense when they become solid, water is most dense at +4 C and becomes less dense when it freezes or becomes solid. This is not the only uniqueness of water; strangely enough, water in the atmosphere doesn’t normally freeze at 0 C.
For atmospheric water to freeze, it needs something to freeze onto. Just like water droplets need something to condense onto, ice crystals need something to freeze onto. The problem is that in the atmosphere there are large numbers of particles for water to condense onto (condensation nuclei) but very few particles for water to freeze onto (ice nuclei).
For ice to form at temperatures just below 0 C, you need a six-sided structure, and there are not many of these around. Ice crystals themselves are six-sided, but where do you get the ice crystal in the first place?
Because of this, if the cloud temperature is warmer than -4 C, the cloud will be made up of super-cooled water. If we cool the cloud down to around -10 C, ice crystals will begin to form even if there are no ice nuclei, so at these temperatures the cloud will consist of a mixture of ice crystals and super-cooled water.
The super-cooled water that falls from these clouds will freeze as soon as it hits a cold surface. Once temperatures fall to -30 C, the cloud will consist almost entirely of ice crystals; below -40 C, the whole cloud will be made up of ice crystals.
So within cold clouds we usually have a combination of ice crystals and super-cooled water.
In warm clouds, rain develops through the process of collision and coalescence; tiny cloud droplets blow around and collide and grow together until they are big enough to fall to the ground. It takes a lot of collisions as a cloud droplet is about 1/100 the size of a small raindrop. Luckily there are a lot of cloud droplets.
In a cold cloud, we have a similar process but first another process has to work its magic: the Bergeron process.
The Bergeron process relies on another unique property of water. If there is just enough water vapour in the air to keep a super-cooled water droplet from evaporating, there is more than enough water vapour in the air for an ice crystal to grow larger. This is because the saturation vapour pressure over ice is lower than that over water. That means ice crystals will attract water vapour more readily than water droplets will.
So our cold cloud now has ice crystals growing and pulling water vapour from the atmosphere. As the amount of water vapour in the atmosphere drops, our super-cooled droplets begin to evaporate to help make up the difference. These droplets evaporate and the ice crystals continue to grow at the expense of the super-cooled water droplets.
After a while, the cloud consists mostly of ice crystals. This process by itself would only result in light amounts of precipitation. For heavier precipitation, we need the second process to kick in: riming and aggregation.
But that’s a topic for a subsequent article.
Source: Farmtario.com